
Bond angles (BA) – The angle between two adjacent bonds in the same atom. Molecular geometry – The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). What is the electron domain geometry?Įlectron domain geometry – The arrangement of electron domains surrounding the central atom of a molecule or ion. They differ as molecular geometry refers to the arrangement of atoms in a molecule around the central atom(s), while electron geometry refers to the arrangement of electron density around the central atom(s). The definitions of molecular geometry and electronic geometry are different. What is electron geometry vs molecular geometry? Does BrF3 have 120 bond angles?īrF3 Bond Angle BrF3 has a T-shaped or Trigonal Bipyramidal molecular geometry, with a bond angle of 86.2 °, which is somewhat less than the typical 90°. The bond angle of the BrF3 is not around 120 degrees, so technically, it is not a trigonal planar. BrF3 has single pairs at the center atom, and for a compound to be trigonal planar, the center atom should not have a lone pair. Is BrF3 trigonal planar? No, BrF3 is not a trigonal planar.

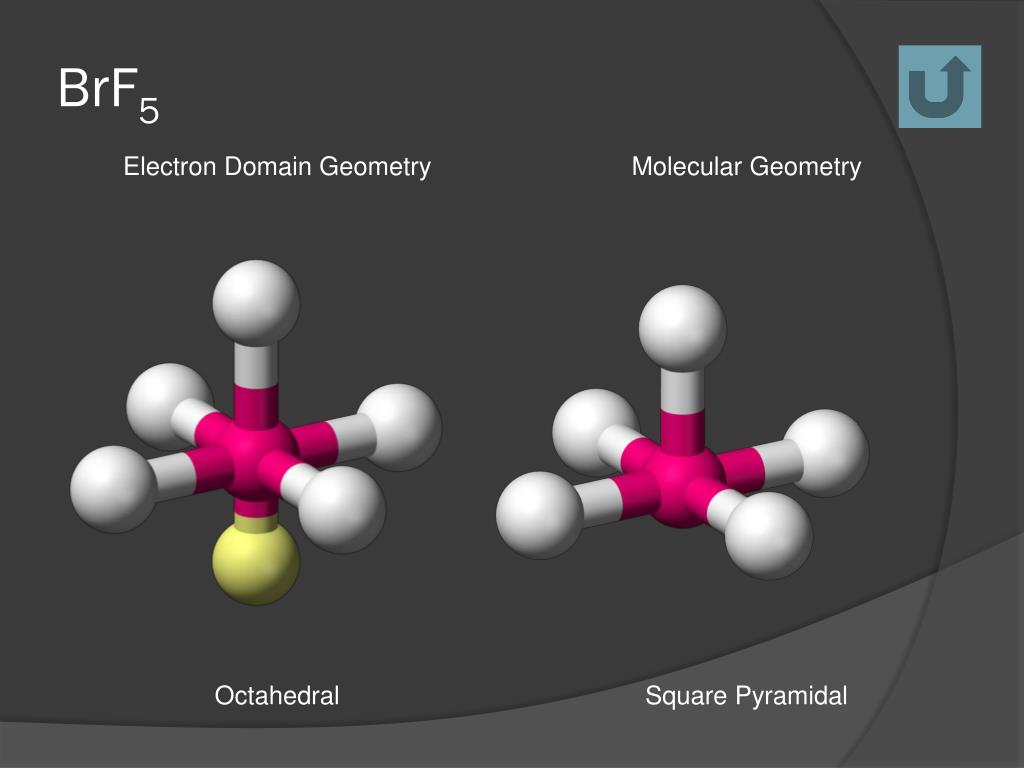
The sp3d2 hybridization of the BrF5 molecule is formed when one S orbital, three p orbitals, and two d orbital join together to form the BrF5 molecular orbital. The bromine and fluorine atoms have s,p and d orbitals. Does BrF5 have a central atom with sp3d2 hybridization? A core bromine atom is surrounded by five fluorides and a pair of electrons in this molecule. See also Is 32C or 34B bigger? Is BrF5 polar or non polar?īecause of its square pyramidal molecular structure, asymmetric charge distribution, and 90° bong angle, bromine pentafluoride (BrF5) is a polar molecule. This angle formed due to the repulsion generated by the electron pairs which is greater than that of the Br-F bonds. Why is it important to know the molecular geometry?īrF3 Molecular Geometry And Bond Angles BrF3 molecular geometry is said to be T-shaped or Trigonal Bipyramidal with a bond angle of 86.2o which is slightly smaller than the usual 90°.What are the different molecular geometry?.How do you find the electron domain geometry and molecular geometry?.Which molecule is having same electronic geometry as well as molecular geometry?.Which of the following species has 120 bond angles *?.


 0 kommentar(er)
0 kommentar(er)
